How is CF treated?
Management and treatment of CF is lifelong and ongoing. The severity of CF symptoms can vary from person to person, so treatments are tailored to each individual.
Treatment plans are prepared by specialist CF physicians in consultation with the person with CF and their family. People with CF generally visit a hospital CF clinic several times each year so their progress can be monitored.
Daily routines for people with CF will vary, but the most common treatments are:
- Intensive daily chest physiotherapy, and other airway clearance techniques, to loosen mucus from the lungs
- Taking enzyme replacement capsules with food to aid digestion
- Antibiotic therapy to treat lung infections
- Aerosol mist inhalations via a nebuliser to help open the airways
- Salt and vitamin supplements
- A nutritious diet that’s also high calorie, high salt and high fat
- Exercise which is important to help clear the airways and build core strength
- CF medications, such as CFTR modulators.

What are CFTR modulators?
In recent years, a new type of medication, known as CFTR modulators, has become available in Australia for people with CF. CFTR modulators work differently to other medications for CF. They work by correcting the malfunctioning protein that causes CF. In doing so, these medications directly address the cause of CF, rather than just the symptoms. They are not a cure, but they help the body’s cells to function more normally.
Cystic fibrosis is caused by changes in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. This gene controls the production of CFTR protein. The CFTR protein creates channels on the cell surface to allow the movement of chloride in and out of the cell. When the CFTR protein is not working properly, the balance of chloride and fluids is affected.
This means that for people who have CF with no CFTR protein (or it is not working properly), mucus in various organs becomes thick and sticky. This can lead to infections in the lungs, damage to the pancreas, and problems in other parts of the body.
Most medications for CF focus on treating the symptoms of CF. CFTR modulators work differently. They aim to restore the function of the faulty CFTR protein made by the CFTR gene and treat the cause of CF. However, as there are approximately 1,800 gene changes that cause CF, and different gene changes can cause different defects in the CFTR protein, the CFTR modulators that have been developed so far are only effective in people with specific gene changes.
Currently, the four CFTR modulators that have been approved for use in Australia are:
- Kalydeco® (ivacaftor)
- Orkambi® (lumacaftor/ivacaftor)
- Symdeko® (tezacaftor/ivacaftor)
- Trikafta® (elexacaftor/tezacaftor/ivacaftor).
There are also other potential CFTR modulators being developed and tested in clinical trials.
Kalydeco®
Kalydeco® was the first CFTR modulator approved for use in Australia. The active ingredient in Kalydeco® is called ivacaftor. Ivacaftor binds to the defective CFTR protein at the cell surface and helps to open the channel so that chloride can flow in and out of the cells. This helps the balance of chloride and fluids at the surface of the cells, and helps to thin mucus in the lungs and other organs.
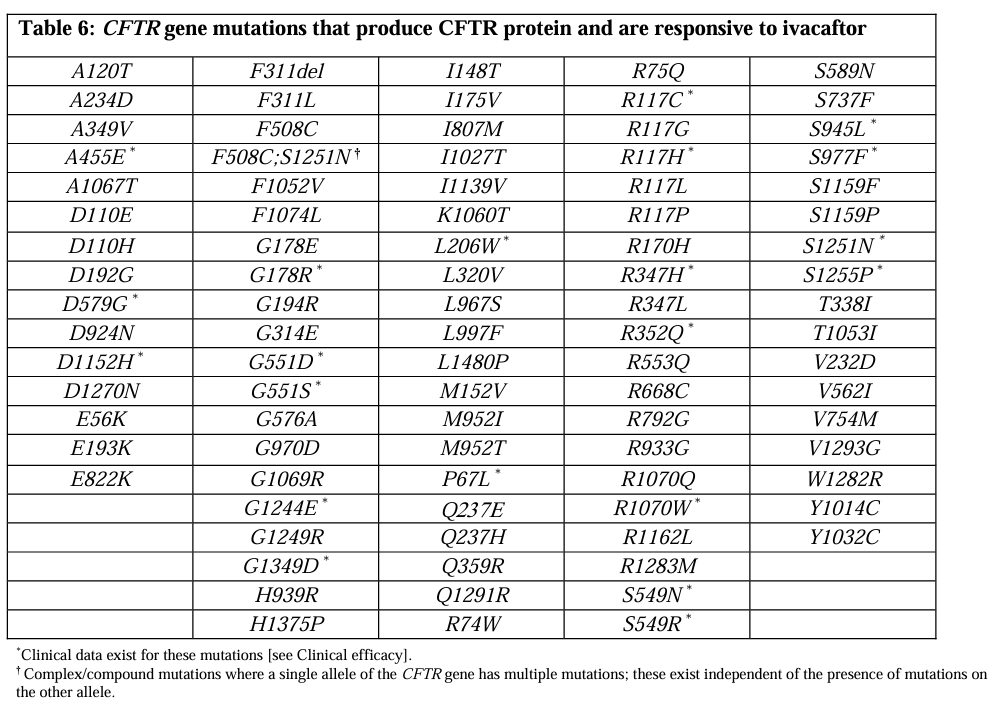
In Australia, the Therapeutic Goods Administration (TGA) has approved the use of Kalydeco®. It is available on the Pharmaceutical Benefits Scheme (PBS) at a government-subsidised price (up to $41 or $6.60 with a concession card) for people who have CF, children aged 4 – 12 months with a gating class III recommendation, plus an additional 92 gene mutations (see table below).
Orkambi®
Orkambi® is a combination therapy. It had two active ingredients, ivacaftor and lumacaftor. Lumacaftor helps the F508del-CFTR protein change to its correct shape, move to the surface of the cell and stay there longer. Ivacaftor then helps to open the channel so that chloride can flow in and out of the cells.
The TGA has approved the use of Orkambi®. The PBS will subsidise the price for people who have CF, are aged two years and older, and who have two copies of the F508del gene change in the CFTR gene.
Symdeko®
Symdeko® is also a combination therapy. It had two active ingredients, ivacaftor and tezacaftor. Tezacaftor works in a similar way to lumacaftor. It helps the CFTR protein change to its correct shape, move to the surface of the cell and stay there longer. Ivacaftor then helps to open the channel so that chloride can flow in and out of the cells.
The TGA has approved the use of Symdeco®. The PBS subsidises the price for people who have CF and are:
- aged 12 years and older, who have two copies of the F508del gene change in the CFTR gene
- aged 12 years and older, who have one copy of the F508del gene change, and one of the following changes in the CFTR gene: E56K, R117C, , S977F, F1074L, 3849+10kbC→T, P67L, E193K, D579G, F1052V, D1152H, R74W, L206W, 711+3A→G, K1060T, D1270N, D110E, R352Q, E831X, A1067T, 2789+5G→A, D110H, A455E, S945L, R1070W, 3272-26A→GIn November 2019, the Pharmaceutical Benefits Advisory Committee (PBAC) recommended that Symdeko® be subsidised by the PBS for people who have CF, are aged 12 years and older, and who have one copy of the following changes in the CFTR gene: E56K, R117C, F508del, S977F, F1074L, 3849+10kbC→T, P67L, E193K, D579G, F1052V, D1152H, R74W, L206W, 711+3A→G, K1060T, D1270N, D110E, R352Q, E831X, A1067T, 2789+5G→A, D110H, A455E, S945L, R1070W, 3272-26A→G.
Trikafta®
Trikafta® is a triple combination therapy. It has three active ingredients, elexacaftor, tezacaftor and ivacaftor. Elexacaftor and tezacaftor help the F508del-CFTR protein change to the correct shape, move to the surface of the cell and stay there longer. Ivacaftor then helps to open the channel so that chloride can flow in and out of the cells.
The TGA has approved the use of Trikafta® and the PBS subsidised the price for people who have CF and are:
- aged 6 years and older, who have at least one copy of the F508del gene change in the CFTR gene
This information is for general purposes. It is not intended to replace advice from your CF clinic team. Different therapies are suited to different people depending on their situation. Speak with your CF clinic team to discuss your specific circumstances and whether these therapies are suitable for you.
More information
References